Cardiovascular Complications of Ophthalmic Timolol As Seen in Patients in South-South Nigeria: Case Series and Literature Review
Main Article Content
Abstract
Background: Timolol, a non-selective beta-blocker, is used in the treatment of Glaucoma. Despite its topical application, reports indicate the occurrence of cardiovascular side effects resulting from systemic absorption. Here, we present three case series illustrating the bradycardic impact of ophthalmic timolol.
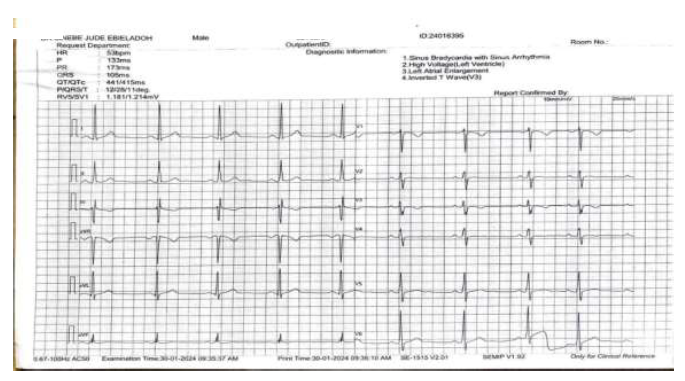
Case Presentations: We report 3 cases of outpatients of Goodheart Medical Consultants Hospital who presented with symptoms such as dizziness and low pulse rate. Electrocardiography indicated a low heart rate of 48,52 and 53 beats per minute respectively. Although bradycardia resolved for the first 2 cases after discontinuing timolol it didn’t resolve for the 3rd case.
Conclusion: Using ophthalmic timolol can trigger bradycardia, with discontinuation not always resolving the condition. Patients prescribed ophthalmic timolol should undergo regular cardiovascular monitoring. Immediate discontinuation of timolol is advised if any adverse effects are observed to prevent potential long-term consequences.
Article Details
References
Mäenpää J, Pelkonen O. Cardiac safety of ophthalmic timolol. Expert Opin Drug Saf. 2016 Nov 1;15(11):1549–61.
Timolol - an overview | ScienceDirect Topics [Internet]. [cited 2024 Feb 18]. Available from: https://www.sciencedirect.com/topics/neuroscience/timolol
Timolol 0.1% in Glaucomatous Patients: Efficacy, Tolerance, and Quality of Life [Internet]. [cited 2024 Feb 18]. Available from: https://www.hindawi.com/journals/joph/2019/4146124/
Von Zup M, Lassaline M, Kass PH, Miller PE, Thomasy SM. Effects of 0.2% brimonidine and 0.2% brimonidine–0.5% timolol on intraocular pressure and pupil size in normal equine eyes. Equine Vet J. 2017;49(6):810–4.
Wójcik-Gryciuk A, Skup M, Waleszczyk WJ. Glaucoma –state of the art and perspectives on treatment. Restor Neurol Neurosci. 2016 Jan 1;34(1):107–23.
Allison K, Patel D, Alabi O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus [Internet]. 2020 Nov 24 [cited 2024 Feb 17]; Available from: https://www.cureus.com/articles/42672-epidemiology-of-glaucoma-the-past-present-and-predictions-for-the-future
Kyari F, Entekume G, Rabiu M, Spry P, Wormald R, Nolan W, et al. A Population-based survey of the prevalence and types of glaucoma in Nigeria: results from the Nigeria National Blindness and Visual Impairment Survey. BMC Ophthalmol. 2015 Dec 12;15(1):176.
Glaucoma Medicines | National Eye Institute [Internet]. [cited 2024 Feb 20]. Available from: https://www.nei.nih.gov/Glaucoma/glaucoma-medicines
Kirwan JF, Nightingale JA, Bunce C, Wormald R. β Blockers for glaucoma and excess risk of airways obstruction: population based cohort study. BMJ. 2002 Dec 14;325(7377):1396–7.
Sidjanin DJ, McCarty CA, Patchett R, Smith E, Wilke RA. Pharmacogenetics of ophthalmic topical β-blockers. Pers Med. 2008;5(4):377–85.
Usifoh SF, Udezi WA. Prescription Patterns and Cost Effectiveness of Antiglaucoma Drugs in a Tertiary Hospital in Nigeria. East Cent Afr J Pharm Sci. 2020 May 20;23(1):36–45.
Pratt NL, Ramsay EN, Kalisch Ellett LM, Nguyen TA, Roughead EE. Association between Ophthalmic Timolol and Hospitalisation for Bradycardia. J Ophthalmol. 2015 Mar 22;2015:e567387.
Gallegos AC, Davis MJ, Tchanque-Fossuo CN, West K, Eisentrout-Melton A, Peavy TR, et al. Absorption and Safety of Topically Applied Timolol for Treatment of Chronic Cutaneous Wounds. Adv Wound Care. 2019 Nov 1;8(11):538–45.
Arbabi A, Bao X, Shalaby WS, Razeghinejad R. Systemic side effects of glaucoma medications. Clin Exp Optom. 2022 Feb 17;105(2):157–65.
Qin F, Zeng L, Zhu Y, Cao J, Wang X, Liu W. Preparation and evaluation of a timolol maleate drug–resin ophthalmic suspension as a sustained-release formulation in vitro and in vivo. Drug Dev Ind Pharm. 2016 Apr 2;42(4):535–45.
Chastain JE. Subchapter 4.2 - Ocular pharmacokinetics. In: Ohia SE, Sharif NA, editors. Handbook of Basic and Clinical Ocular Pharmacology and Therapeutics [Internet]. Academic Press; 2022 [cited 2024 Feb 18]. p. 179–219. Available from: https://www.sciencedirect.com/science/article/pii/B9780128192917000058
Vaajanen A, Vapaatalo H. A Single Drop in the Eye – Effects on the Whole Body? Open Ophthalmol J. 2017 Oct 31;11:305–14.
Abbas SA, Hamadani SM, Ahmad U, Desai A, Kitchloo K. Ophthalmic Timolol and Hospitalization for Symptomatic Bradycardia and Syncope: A Case Series. Cureus [Internet]. 2020 Mar 14 [cited 2024 Feb 21]; Available from: https://www.cureus.com/articles/28877-ophthalmic-timolol-and-hospitalization-for-symptomatic-bradycardia-and-syncope-a-case-series
Rana MA, Mady AF, Rehman BA, Alharthy A, Huwait B, Riaz A, et al. From Eye Drops to ICU, a Case Report of Three Side Effects of Ophthalmic Timolol Maleate in the Same Patient. Case Rep Crit Care. 2015;2015:1–4.
Arbabi A, Bao X, Shalaby WS, Razeghinejad R. Systemic side effects of glaucoma medications. Clin Exp Optom. 2022 Feb 17;105(2):157–65.
Rains J, Kesterson J. Ocular timolol as the causative agent for symptomatic bradycardia in an 89-year-old female. Am J Emerg Med. 2021 Apr;42:263.e5-263.e6.
Attanasio A, Baglio S, Quatrana M, Bartorelli L. Accelerated idioventricular rhythm associated to ophthalmic timolol/dorzolamide solution. Int J Cardiol. 2004 Jun 1;95(2):343–5.
Elikowski W, Fertała N, Zawodna-Marszałek M, Gaca-Wysocka M, Bolewski A. Bradycardia during optical timolol therapy: its delayed remission after timolol discontinuation and unexpected further relapse.
Wang Z, Denys I, Chen F, Cai L, Wang X, Kapusta DR, et al. Complete atrioventricular block due to timolol eye drops: a case report and literature review. BMC Pharmacol Toxicol. 2019 Dec 2;20(1):73.