Microbiological Investigation and Virulence Factor Characterization of Chronic Suppurative Otitis Media in A Nigerian Tertiary Hospital Microbiological Investigation and Virulence Factor
Main Article Content
Abstract
Background: Chronic suppurative otitis media (CSOM) is a common cause of hearing loss with the attendant poor academic performance and low productivity in workplaces.
Objectives: This study investigated the microbial aetiology and associated virulence mechanisms of CSOM in a Nigerian tertiary hospital.
Methods: This was a prospective study of 52 patients diagnosed with CSOM in either one or both ears who had not taken any antibiotics for at least the last seven (7) days. Ear swabs were taken by an otolaryngologist and sent to the Microbiology Department for processing. The specimens were cultured within 30 min of collection and the isolates identified using standard microbiological techniques. Selected virulence properties were characterised.
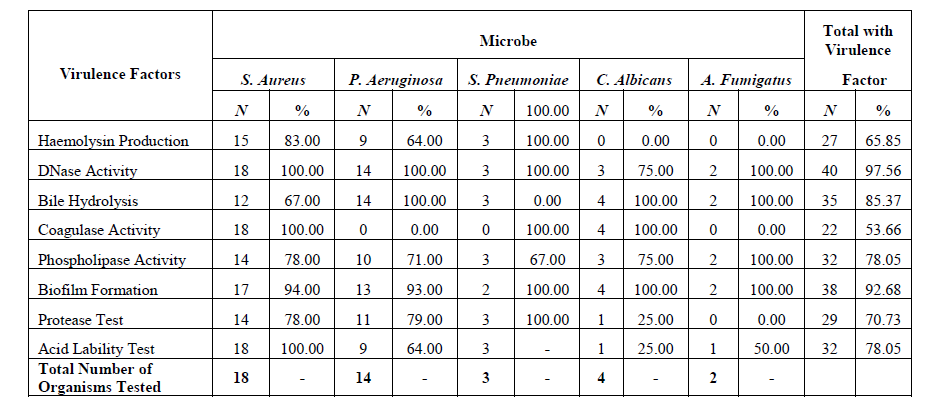
Results: The most common organism isolated was Staphylococcus aureus 18 (34.6%) followed by Pseudomonas aeruginosa 14 (26.9%). Fungal isolates were recovered from 6 11.5%) of specimens; in 11 (21.15%) of cases no microbe was detected. Age did not significantly affect incidence of CSOM of microbial origin (p = 0.1742) although most cases were in the age 31-40 years group. Male and female patients were almost equally affected (M: F = 1:1.08), DNase followed by biofilm formation were the predominant virulence phenotypes identified while coagulase, followed by haemolysin production, were least common.
Conclusion: Staphylococcus aureus and Pseudomonas aeruginosa were the most common organisms among CSOM cases and recovered from all age-groups. Early detection and infection control are key to reducing incidence of CSOM.
Article Details
References
References
Nazir A, Kadri SM. Aerobic bacteriology of chronic suppurative otitis media: A hospital based study. Int J Res Med Sci. 2014;2(4):1521–1525. https://doi.org/10.5455/2320-6012.ijrms20141152
WHO. Chronic suppurative otitis media burden of illness and management options. Geneva, Switzerland: Child and Adolescent Health and Development; Prevention of Blindness and Deafness, World Health Organization; 2004.
Acuin J. Chronic suppurative otitis media. BMJ Clin Evid. 2007;2007:0507.
Wright D, Safranek S. Treatment of otitis media with perforated tympanic membrane. Am Fam Physician. 2009;79(8):650–654.
Minovi A, Dazert S. Diseases of the middle ear in childhood. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2014;13:Doc11. https://doi.org/10.3205/cto000114
Berman S. Otitis media in developing countries. Pediatrics. 1995;96:126–131.
Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by Otitis Media: Systematic review and global estimates. PLoS One. 2012;7(4):e36226. https://doi.org/10.1371/journal.pone.0036226
Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: Beyond the earache. Lancet Infect Dis. 2010;10:195–203. https://doi.org/10.1016/S1473-3099(10)70012-8
Verhoeff M, van der Veen EL, Rovers MM, Sanders EAM, Schilder AGM. Chronic suppurative otitis media: A review. Int J Pediatr Otorhinolaryngol. 2006;70(1):1–12. https://doi.org/10.1016/j.ijporl.2005.08.021
Lasisi AO, Sulaiman OA, Afolabi OA. Socio-economic status and hearing loss in chronic suppurative otitis media in Nigeria. Ann Trop Paediatr. 2007;27(4):291–296. https://doi.org/10.1179/146532807X245689
Adoga A, Nimkur T, Silas O. Chronic suppurative otitis media: Socio- economic implications in a tertiary hospital in Northern Nigeria. Pan Afr Med J. 2010;4:3. https://doi.org/10.4314/pamj.v4i1.53613
Juyal D, Negi V, Sharma M, Adekhandi S, Prakash R, Sharma N. Significance of fungal flora in chronic suppurative otitis media. Ann Trop Med Public Health. 2014;7:120–123.
Khaing TZ, Myint KS, Mu KK, Maw WW. Common clinical spectrum, risk factors and fungal isolates of otomycosis. Myanmar Health Sci Res J. 2016;28(2):113–120.
Pavia AT. Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(Suppl 4):S284–S289. https://doi.org/10.1093/cid/cir043
Marom T, Nokso-Koivisto J, Chonmaitree T. Viral–bacterial interactions in acute Otitis Media. Curr Allergy Asthma Rep. 2012;12(6):551–558. https://doi.org/10.1007/s11882-012-0303-2
Barrow GI, Felham RKA, editors. Cowan and steel’s manual for the identification of medical bacteria. London: Cambridge University Press; 1993.
Narayana Moorthy A, Narasaraju T, Rai P, et al. In vivo and in vitro studies on the roles of neutrophil extracellular traps during secondary pneumococcal pneumonia after primary pulmonary influenza infection. Front Immunol. 2013;4:56. https://doi.org/10.3389/fimmu.2013.00056
Manns JM, Mosser DM, Buckley HR. Production of a hemolytic factor by Candida albicans. Infect Immun. 1994;62(11):5154–5156.
Kumar CPG, Kumar SSJ, Menon T. Phospholipase and proteinase activities of clinical isolates of Candida from immunocompromised patients. Mycopathologia. 2006;161:213. https://doi.org/10.1007/s11046-005-0157-4
Mobarak-Qamsari E, Kasra-Kermanshahi R, Moosavi-nejad Z. Isolation and identification of a novel, lipase-producing bacterium, pseudomnas aeruginosa KM110. Iran J Microbiol. 2011;3(2):92–98.
Mageswari A, Karthikeyan S, Anbalagan M, Sivakumar A, Gothandam KM. Screening and optimization of protease production from a halotolerant Bacillus licheniformis isolated from saltern sediments. J Genet Eng Biotechnol. 2013;11:47–52. https://doi.org/10.1016/j.jgeb.2013.02.002
Christensen GD, Bisno AL, Simpsom WA, Beachey EH. Adherence of slime producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326.
Yigit N, Aktas AE, Ayyildiz A. Detection of coagulase activity in pathogenic Candida species. J Int Med Res. 2008;36(6):1378–1382. https://doi.org/10.1177/147323000803600627
Edberg SC, Gallo P, Kontnick C. Analysis of the virulence characteristics of bacteria isolated from bottled, water cooler, and tap water. Microbial Ecol Health Dis. 1996;9(2):67–77. https://doi.org/10.3109/08910609609166445
Oli AN, Okoli KC, Ujam NT, Adje DU, Ezeobi I. Health professionals’ knowledge about relative prevalence of hospital-acquired infections in Delta State of Nigeria. Pan Afr Med J. 2016;24:148. https://doi.org/10.11604/pamj.2016.24.148.9270
Ejiofor SO, Edeh AD, Ezeudu CE, Gugu TH, Oli AN. Multi-drug resistant acute otitis media amongst children attending out-patient clinic in Chukwuemeka Odumegwu Ojukwu University Teaching Hospital, Awka, South-East Nigeria. Adv Microbiol. 2016;6:495–501. https://doi.org/10.4236/aim.2016.67049
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG, Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):606–663. https://doi.org/10.1128/CMR.00134-14
Pechère JC, Köhler T. Patterns and modes of β-lactam resistance in Pseudomonas aeruginosa. Clin Microbiol Infect. 1999;5(1):S15–S18.
Sibhghatulla S, Jamale F, Shazi S, Syed M, Danish R, Amjad KM. Prevalence of multidrug resistant and extended spectrum beta-lactamase producing Pseudomonas aeruginosa in a tertiary care hospital. Saudi J Biol Sci. 2015;22:62–64. https://doi.org/10.1016/j.sjbs.2014.06.001
Osazuwa F, Osazuwa E, Osime C, et al. Etiologic agents of otitis media in Benin city, Nigeria. North Am J Med Sci. 2011;3(2):95–98. https://doi.org/10.4297/najms.2011.395
Mansoor T, Musani MA, Khalid G, Kamal M. Pseudomonas aeruginosa in chronic suppurative otitis media: Sensitivity spectrum against various antibiotics in Karachi. J Ayub Med Coll Abbottabad. 200;21(2):120–123.
Shamweel A. Antibiotics in chronic suppurative otitis media: A bacteriologic study. Egypt J Ear Nose Throat Allied Sci. 2013;14:191–194. https://doi.org/10.1016/j.ejenta.2013.06.001
Kim SH, Kim MG, Kim SS, Cha SH, Yeo SG. Change in detection rate of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa and their antibiotic sensitivities in patients with chronic suppurative otitis media. J Int Adv Otol. 2015;11(2):151–156. https://doi.org/10.5152/iao.2015.1106
Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 2005;1(1):e1. https://doi.org/10.1371/journal.ppat.0010001
Ibekwe TS, Nwaorgu OGB. Classification and management challenges of otitis media in a resource-poor country. Niger J Clin Pract. 2011;14(3):262–269. https://doi.org/10.4103/1119-3077.86764
Fasunla AJ, Samdi M, Nwaorgu OG. An audit of ear, nose and throat diseases in a tertiary health institution in South-western Nigeria. Pan Afr Med J. 2013;14:1. https://doi.org/10.11604/pamj.2013.14.1.1092
Oli Angus N, Akabueze Vivian B, Ezeudu Chijioke E, et al. Bacteriology and antibiogram of urinary tract infection among female patients in a tertiary health facility in South Eastern Nigeria. Open Microbiol J. 2017;11:292–300. https://doi.org/10.2174/1874285801711010292
Qi L, Li H, Zhang C, et al. Relationship between antibiotic resistance, biofilm formation, and biofilm-specific resistance in Acinetobacter baumannii. Front Microbiol. 2016;7:483. https://doi.org/10.3389/fmicb.2016.00483
Singh S, Singh SK, Chowdhury I, Singh R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J. 2017;11:53–62. https://doi.org/10.2174/1874285801711010053
Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4(2):10.1128/microbiolspec.VMBF–0016–2015. https://doi.org/10.1128/microbiolspec.VMBF-0016-2015
Thomer L, Schneewind O, Missiakas D. Pathogenesis of Staphylococcus aureus bloodstream infections. Ann Rev Pathol. 2016;11:343–364. https://doi.org/10.1146/annurev-pathol-012615-044351
McAdow M, Missiakas DM, Schneewind O. Staphylococcus aureus secretes coagulase and von Willebrand factor binding protein to modify the coagulation cascade and establish host infections. J Innate Immun. 2012;4(2), 141–148. https://doi.org/10.1159/000333447
Xie J, Juliao PC, Gilsdorf JR, Ghosh D, Pate M, Marrs CF. Identification of new genetic regions more prevalent in nontypeable Haemophilus influenza otitis media strains than in throat strains. J Clin Microbiol. 2006;44(12):4316–4325. https://doi.org/10.1128/JCM.01331-06
Hallbauer UM, Atkins MD, Tiedt NJ, Butler IRT, Pieters M, Elliott E, Joubert G, Seedat RY. Co-morbidities in children presenting with chronic suppurative otitis media – A South African study. J Trop Pediatr. 2014;60(3):198–202. https://doi.org/10.1093/tropej/fmt107
Mulcahy H, Charron-Mazenod L, Lewenza S. Pseudomonas aeruginosa produces an extracellular deoxyribonuclease that is required for utilization of DNA as a nutrient source. Environ Microbiol. 2010;12:1621–1629.
Mann EE, Rice KC, Boles BR, et al. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One. 2009;4:e5822. https://doi.org/10.1371/journal.pone.0005822
Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–259. https://doi.org/10.1194/jlr.R500013-JLR200
Mohan das V, Ballal M. Proteinase and phospholipase activity as virulence factors in Candida species isolated from blood. Rev Iberoam Micol. 2008;25(4):208–10.
Pavlickova S, Klancnik A, Dolezalova M, Mozina SS, Holko I. Antibiotic resistance, virulence factors and biofilm formation ability in Escherichia coli strains isolated from chicken meat and wildlife in the Czech Republic. J Environ Sci Health B. 2017;52(8):570–576. https://doi.org/10.1080/03601234.2017.1318637
Sun Y, Wilkinson BJ, Standiford TJ, Akinbi HT, O’Riordan MXD. Fatty acids regulate stress resistance and virulence factor production for Listeria monocytogenes. J Bacteriol. 2012;194(19):5274–5284. https://doi.org/10.1128/JB.00045-12
Charland N, Kobisch M, Martineau-Doizé B, Jacques M, Gottschalk M. Role of capsular sialic acid in virulence and resistance to phagocytosis of Streptococcus suis capsular type 2. FEMS Immunol Med Microbiol. 1996;14(4):195–203. https://doi.org/10.1111/j.1574-695X.1996.tb00287.x
Schrager HM, Rheinwald JG, Wessels MR. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Invest. 1996;98(9):1954–1958. https://doi.org/10.1172/JCI118998
Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev. 2004;68(1):132–153. https://doi.org/10.1128/MMBR.68.1.132-153.2004
Oli AN, Nweke JN, Ugwu MC, Anagu LO, Oli AH, Esimone CO. Knowledge and use of disinfection policy in some government hospitals in South-East, Nigeria. Br J Med Med Res. 2013;3(4):1097–1108. https://doi.org/10.9734/BJMMR/2013/2478